Iron and manganese incrustations > 90%
pH neutral reducing agents remove iron and manganese incrustations 50 x more efficiently than acids with pH value 1,0 in identical molar concentration, i.e. 100 g iron(II) compared with 2 g.
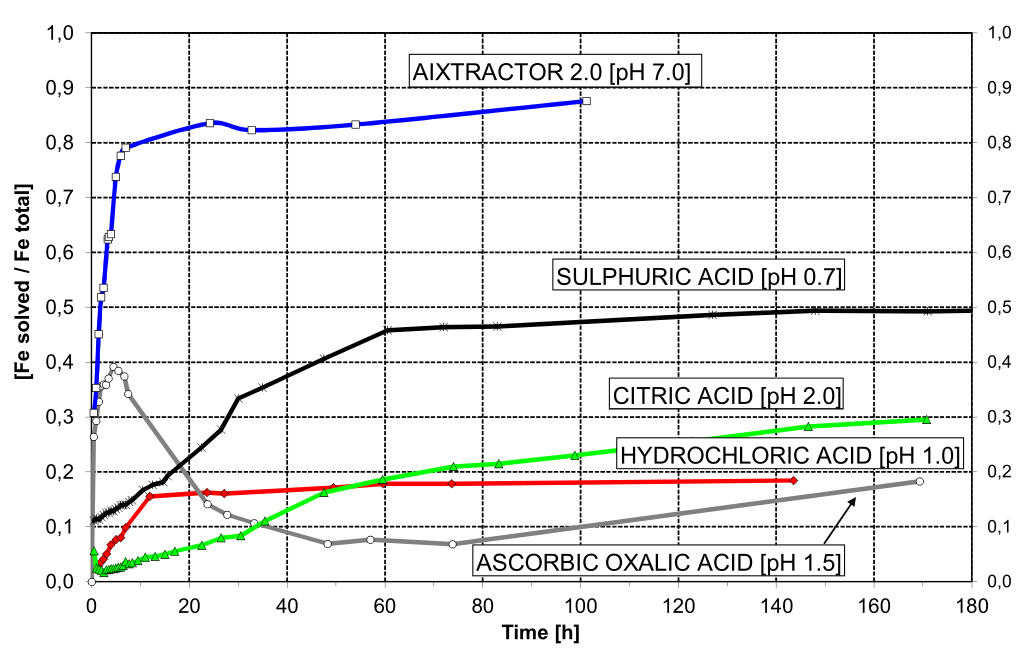
(Houben 1998)
German technical standard DVGW W 130 specifies that any rehabilitation agent should have:
- a high dissolution capacity within an acceptable period of time
- a cost-effective ratio between the dissolved incrustations and the price of the required amount of agent
A successful chemical rehabilitation is based on the identification of the incrustations to be solved.
The agent must not have a corrosive impact on well lining materials or damage the calcium and silicium minerals of the gravel pack and the aquifer. Toxic (e.g. titanium) substances and those that cause eutrophication (e.g. ascorbic or citric acids) are not to be applied either.
Selection criteria of chemical agents
| Nature of the geological formation | chemical procedure | |
|---|---|---|
| acidic agents (with pH-regulation) | pH-neutral agents | |
| Solid rock, limy, marly (karst and joint aquifiers) | - | + |
| Solid rock, sandstones without limy binder (joint aquifier) | 0 | + |
| Solid rock, crystalline (joint aquifiers) | 0 | + |
| Loose rock, limy, marly (molasses, slightly consolidated chalk sands etc.) | - | + |
| Loose rock, quartzous (terrace gravel without lime components) | 0 | + |
| Loose rock, quartzous (terrace gravel with lime components) | - | + |
| + | suitable as per individual adjustment according to well lining materials, gravel pack and mineralogy of the aquifer |
| - | unsuitable due to a danger of dissolution of calcium and its precipitation in the aquifer |
| 0 | conditionally suitable, risk of dissolution of silicates due to the low pH values |
(Treskatis 2002)








 » Calculate here
» Calculate here » Have fun!
» Have fun!